Nanotoxicity
- Home
- RESEARCH
- Nanotoxicity
Nanotoxicity
An emerging discipline, nanotoxicology provides information about the potential toxicological effects of nanomaterials on human health, their interaction with biological systems, and their risk assessment. Nanotoxicology is defined as “science of engineered nanodevices and nanostructures that deals with their effects in living organisms ”. Nanotoxicology elucidates the dose-response relationships between physicochemical properties of nanomaterials and toxic effects on biological system.
Moreover, recent growing interest in the uptake mechanism and trafficking of nanoparticles at cellular and systemic levels raises concern about their toxicity and biocompatibility. Some nanoparticles are massively taken up by cells through endocytosis, and they possess the capacity to penetrate barriers such as vascular endothelium and endothelial cells. This leads to easy uptake of nanoparticles by the human body compared to large particles, subsequently gaining access to cells, tissues, and organs. Nanotoxicology provides information about the physicochemical properties of nanomaterials and adverse effects on biological systems, since the toxicity of nanoparticles is not easily predictable from the known properties of larger bulk materials. Furthermore, nanomaterials used in medicine, pharmaceutics, cosmetics, and food are intensively designed to enhance cellular interaction and delivery of active molecules to target organs by controlling particle size and modifying surface charge and physicochemical properties.
However, there are currently no general guidelines to assess the potential risk of nanoparticles and no regulatory framework for their human-related applications. Therefore, more vigorous investigations on the potential toxicity and safety assessment of nanoparticles are urgently needed to establish minimum safety standard. Such toxicological studies provide critical information on the medical applications of nanoparticles and contribute to the sustainable development of nanotechnology with safe and biocompatible levels.
Toxicity in vitro
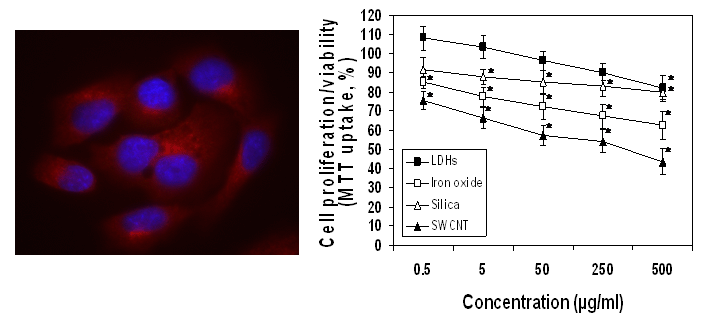
The first step towards understanding how a nanomaterial will react in the body often involves in vitro cell culture studies. Compared to animal studies, cellular testing is les ethically ambiguous, is easier to control and reproduce, and is less expensive. In the case of cytotoxicity , controlling the experimental conditions is crucial to ensure that the measured cell death corresponds to the toxicity of the exposed nanoparticles versus the unstable culturing conditions. In addition, as nanoparticles can adsorb dyes and be redox active, it is important that the cytotoxicity assay is appropriate.
Toxicity in vivo
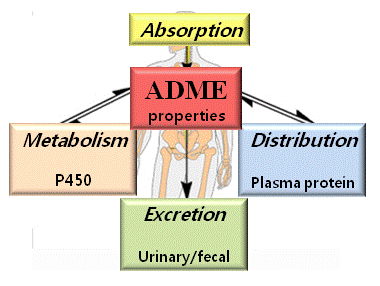
The majority of nanotoxicity research uses in vitro cell culture systems, requiring further verification in vivo complicated systems. In vitro cell culture models do not account for intercellular communication and exclude lymph, blood and bile transport mechanisms. Moreover, the correlation between in vitro and in vivo effects is not always consistent, requiring in vivo experiments to assume the toxicity obtained by cell culture systems. We perform toxicokinetic study (absorption, distribution, metabolism, excretion) and acute or sub-acute toxicity study in animal models.